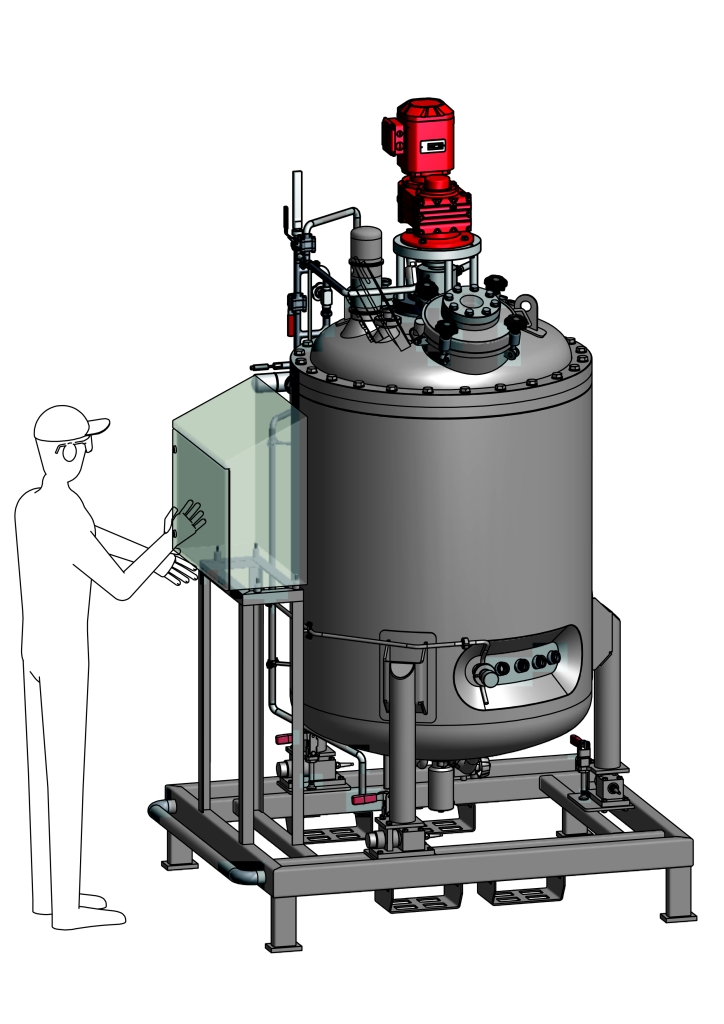
Plant technology, Pharmaceutical, Biotech
Skids and modules for pharmaceutical and biotech applications
for optimum process control and transport
We are experts in the design, engineering and production of complete skids, modules, assemblies and plant components according to your requirements. KASAG holds all necessary certifications (AD-2000, EN13445, China Stamp, ASME, GOST, etc.). We offer comprehensive services. If required, we’ll also procure the defined purchased parts and carry out non-destructive testing on the finished products before acceptance. In addition, we prepare all necessary documents with respect to GMP (Good Manufacturing Practice) requirements and for the validation and qualification procedures (DQ, IQ, OQ, PQ) for the manufacture of pharmaceutical and biotechnology products.
Thanks to our range of approvals ‒ comprising PED (EN13445 / AD-2000), ASME (U-Stamp, Code Section VIII Div. 1), China Stamp (A2), China License, TP TC 032/2013 (EAC) customs union ‒ we offer plant technology for your production plants in almost all countries of the world.